Background
The controlled synthesis of functional organic macromolecules is an important topic in the areas of materials science and chemical biology. After continuous development in the recent decades, polymeric materials in micrometer to nanometer size have shown great potential in targeted delivery, recognition, triggered response and release, biocatalysis and multivalent enhancement. In addition, the emergence of various living polymerization strategy has significantly facilitated the controlled synthesis of functional polymers, making it much easier for effecting chemistry for macromolecules with desired functional moieties. By combining known synthetic strategies and molecular biology mechanisms, rationally designed novel macromolecules can be used as catalytic, antimicrobial, delivery or targeting moieties, or as tools for studying complicated mechanisms in living organisms. Useful as it is, such design and application has become a frontier in materials science and chemical biology. As researchers and higher education providers, we want to design new strategies for the several aims listed below, trying to promote the application of nano, polymeric or protein-based materials in chemical biology and medicine, at the same time training new generations of scientists with good skill, innovation and knowledge in this area.
功能化有机高分子的可控合成是现代材料科学和化学生物学的一个重要课题。在几十年的发展后,纳米到微米尺度的高分子材料已经在靶向运输、特异性识别、刺激响应、可控释放、生物催化、多位点增强效应(multivalent effect)等方面显示出了巨大的潜力。另一方面,各种高分子活性聚合方法(living polymerization)的提出与发现也大大方便了功能化有机高分子的可控合成,使得具有特定活性官能团或基团的高分子更容易在化学层面上实现。通过结合已知的化学合成方法和已知的分子生物学机理,合理设计(rational design)新型的功能化高分子来实现可预期的高效催化、杀菌、输送、识别等效果,或者使用此类高分子材料作为工具来研究更深层次的生命科学机理,已经成为材料科学和化学生物学的热门与前沿领域。由此,本课题组希望借助高分子聚合方法学、有机合成、纳米材料合成和化学生物学方面的已有的知识,针对以下几个特定的研究课题制定方法以实现预期目标,并同时推进天然与人工高分子材料在化学生物学和医学上的应用的研究,并在研究的同时培养出新一批在该领域具备扎实理论功底和创新研究能力的科技人才。
Artificial Metalloenzymes
In all the life forms, enzymes of different structures and functions are essential. By catalyzing different biochemical reactions, they keep metabolism running and sustain life. Enzymes are basically proteins formed from the folding of polypeptide chains, and are called metalloenzymes if metal cofactors are incorporated into the scaffolds. Inside enzymes are hydrophobic cavities, which can selectively bind substrates near the active metal centers, effecting catalytic process and forming the corresponding product with high selectivity and activity. As we can see here, this is essentially a metal-organic catalyst “wrapped” by a polymeric ligand, which provides selectivity over substrates and protection on the metal centers, making this combination so powerful that it can perform highly efficient catalysis in the complicated biological environment.
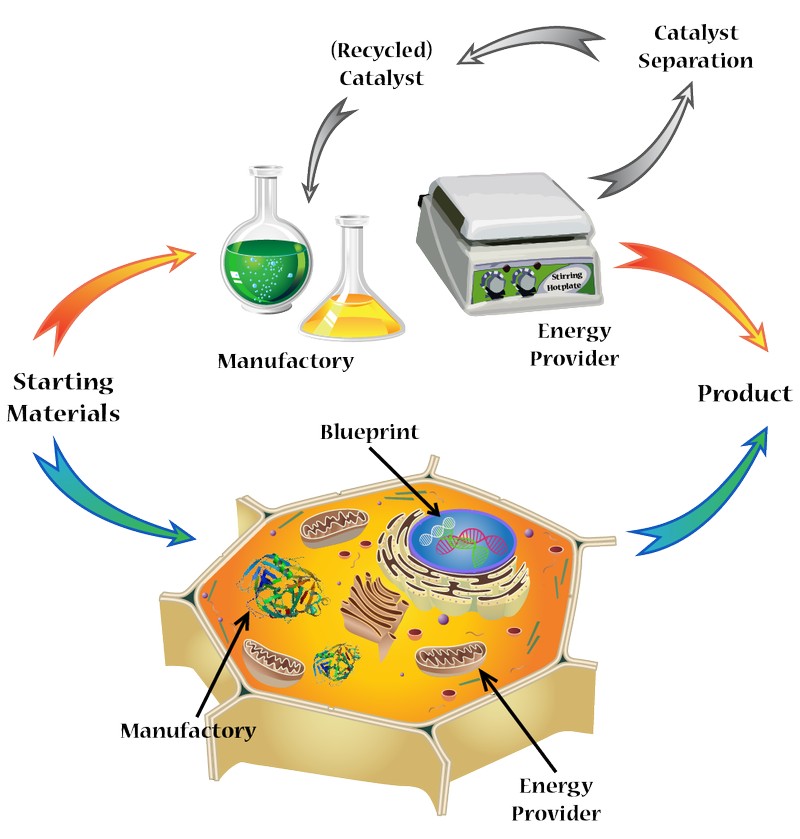
However, enzymatic reactions are “natural” reactions. Although many decades have passed since Wöhler could artificially make urea that thought to be unobtainable without the mysterious “vital force” and ended the hypothesis of vitalism, people are still unable to perform most reactions as efficient as nature does with enzymes. Many important reactions in chemical and pharmaceutical industry are still so “unnatural”, requiring complicated synthetic and purification processes. Despite the large amount of metal-organic and organometallic catalysts reported and used, there are remarkably few examples that can be used in biological environment (aqueous, neutral pH, with the presence of common biomolecules), and even fewer, if existing at all, cases that can be comparable with enzymes in activity and selectivity.
So what if we combine enzyme and synthetic metal catalyst? It can be a good idea but difficulties are many, too. By combining the two we may achieve: 1) metal-organic or organometallic catalysts that work in aqueous environment with high efficiency, which greatly reduces cost and promotes “greenization” of industrial processes; 2) artificial metalloenzymes that can assist or replace some natural enzymes, in order to “repair” some problematic biochemical processes; 3) artificial “synthetic machines” that work inside cells or tissues to catalyze “unnatural” reactions, which can be very useful as tools in chemical biology and medicinal chemistry. Although the three aims look markedly different from each other, they share the same difficulty in getting high activity, biocompatibility and stability simultaneously. Some researchers have started trying to introduce artificial metal cofactors into natural protein scaffolds and perform directed evolution as a process of structure optimization. This is clearly learning from the mother nature, in hope to utilize the polypeptide scaffold to enhance the existing catalysts with an enzymatic working style.
In our lab, artificial metalloenzymes with different functions are prepared to expand the natural enzymatic repertoire with abiotic reactions. As it is known that the sensitivity of metal centers and the resulting low activity and turnover of such artificial metalloenzymes in vivo are systematic problems not fully solved, we are actively seeking for potential solutions to this problem by creating microenvironments inside cells, so that artificial metalloenzymes can enjoy benign media in which they can safely do their catalysis job. For example, liquid-liquid phase separation of in cellulo-assembled artificial metalloenzymes can be created inside Escherichia coli to form membraneless, isolated compartments, so that these metalloenzymes can perform intracellular catalysis with spatial separation. Such engineered Escherichia coli can work as whole-cell catalyst with confined metal species, with potential applications in fields such as non-natural metabolism, fermentation and drug delivery.
在地球上的各种生命形态中,各种各样的酶都扮演着不可或缺的作用。它们各司其职,高效催化各种各样的生物化学反应,保证了生命体的新陈代谢可以持续不断地进行。酶的本质是蛋白质,由多肽链折叠后形成高级结构并结合一个或多个金属离子活性中心而形成。酶的内部有疏水空腔结构,可选择性的结合底物于金属活性中心附近,发生催化过程从而产生化学反应生成相应产物,具有高度选择性和活性。本质上,这是一个高分子配体包裹的金属活性中心,由于不同高分子结构提供的底物选择性和对金属催化中心的保护作用,使得这一组合可以在复杂的生物环境中实现各种强大的催化功能。
从1828年维勒成功使用无机原料第一次人工合成有机物尿素到现在,时间已经过去了190年。尽管这个经典的实验终结了风行一时的、认为有机物存在生命力因而不能通过人工方法从无机物合成的“生命力学说”,人类随后也了解到了这份“生命力”的背后是种种神奇的酶催化反应,但合成有机物的方法在百余年的发展后却仍然远远无法与酶催化合成的效率相比。有机化学中常见的各种金属有机催化剂虽然种类繁多,但可以在生物环境下(水相、中性环境、有常见生物小分子存在等)催化反应的催化剂却非常少见,能达到酶一般的选择性和活性的更是屈指可数。在另外一方面,酶催化的反应虽然多种多样,但仍然是有限的。许多在工业界、制药行业等非常重要的反应在生物化学过程中并不存在,因而也没有对应的酶可以利用。如果能将酶和现有的金属有机催化剂结合起来,便有可能达到三个效果:其一,可能得到具有高活性、水相工作的金属催化剂,实现工业过程的高效化和绿色化;其二,人工催化剂可能可以取代或协助一些天然酶的作用,达到修复某些生物化学过程的目的;其三,作为“微型合成机器”可以在活生物体内催化一些非天然的反应,这将使它们成为药学和化学生物学中非常重要的一类工具,进行原位蛋白质激活或修饰、原位药物合成等。这三个目标效果虽然截然不同,但其实现的方法却可能是类似的,所面对的困难也是类似的。对金属催化剂自身的基础催化性能、生物兼容性和稳定性的要求在客观上为这类仿酶大分子催化剂的合成与应用增添了很大的困难。一些研究者已经开始尝试利用蛋白质骨架加入人工金属催化中心来实现“进化式”催化筛选,试图借鉴大自然的做法,利用酶的机理来指导新型催化剂的设计与合成。
在我们课题组的工作中,我们会尝试组装具有不同功能的人工金属酶,并由此实现天然酶促反应的拓展。然而,我们都知道人工金属酶所含有的金属活性中心一般比较敏感,在胞内工作时往往会出现活性大幅降低(甚至失活)、转换数低等一系列悬而未决的问题,因此我们同时也在尝试在细胞内为人工金属酶建立专属的微环境,令它们能够在这些较为友好的微环境当中稳定工作。例如,我们可以利用液-液相分离技术在大肠杆菌内创制出由人工金属酶构成的无膜细胞器类似物,这样人工金属酶就可以在这些人工细胞器内实现空间可控的催化过程。这些基于大肠杆菌的工程菌可以作为整细胞催化剂来使用,它们可以有效控制重金属催化剂的泄漏,有望在非天然生化过程的建立、非天然发酵过程和药物递送等领域得到很好的应用。
Exogeneous catalytic systems for intracellular abiotic reactions
Intracellular catalysis has the potential to be one of the most important tool in the field of chemical biology. Highly efficient intracellular catalysis can activate an unnatural reaction in a specific location inside cells, affording a functional molecule on demand. For example, if a macromolecular catalyst has selectivity when entering cells, then a anticancer compound may be synthesized only inside cancer cells. Some molecules, small or large, may be synthesized inside cells directly to avoid possible difficulties in introducing them into the cells. Since the concept of intracellular catalysis was announced in 2006 by Streu and Meggers using a ruthenium catalyst as an example, this field has been advancing with considerable difficulties in expanding catalyst pool, concentration limitation, reactivity, biocompatibility and so on. We want to combine the knowledge from chemical biology, organic chemistry and polymer chemistry to seek a solution for the problems in intracellular catalysis.
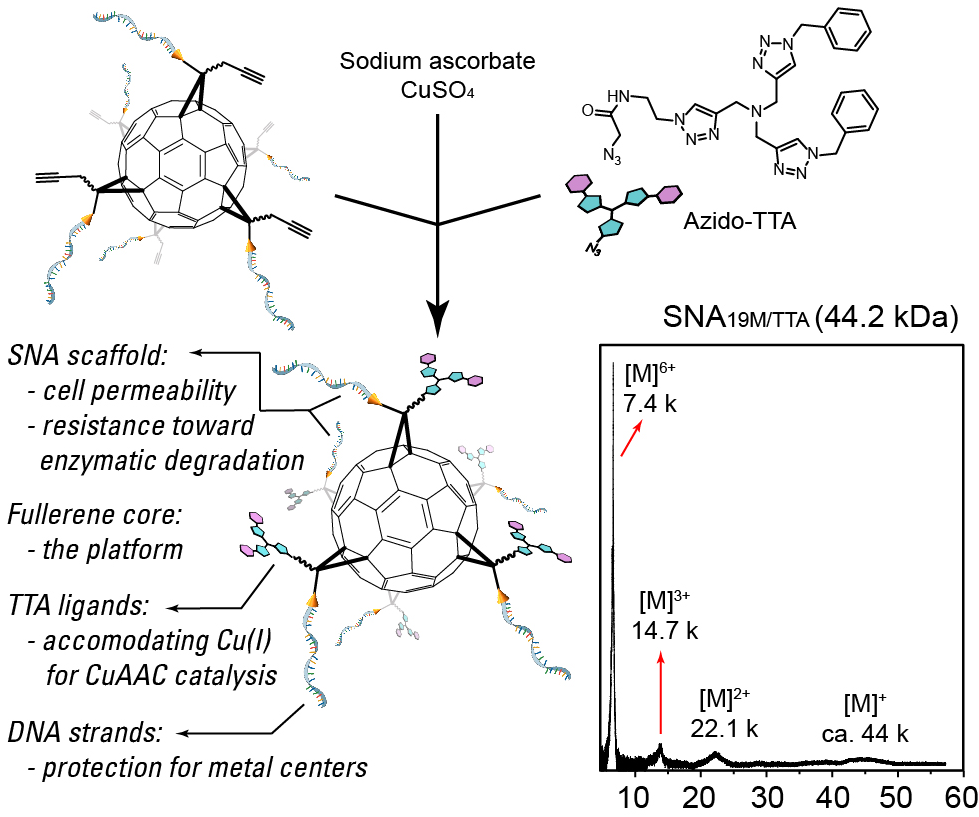
In short, we hope to generate enzyme-mimicking structures, through either organic/polymer synthesis or self-assembly, followed by the combination with artificial metal cofactors. Inspired by nature, such artificial catalysts, called synthetic enzyme mimics, can bring solubility and biocompatibility from their hydrophilic exterior and a stable, highly efficient catalytic center from their hydrophobic cavities in the interior, which may lead to a system that allows changes in catalytic centers while keeping the artificial skeleton to give a multifunctional catalytic platform. We currently have three very different intracellular catalytic systems under development in the lab, namely dense-shell nanoparticle (DSNP) system, spherical nucleic acid (SNA) system, and membrane-anchored catalysis (MAC) system. These systems are all highly efficient exogeneous systems, and each has unique advantages in different application scenarios.
胞内原位催化反应有潜力成为化学生物学领域中最重要的工具之一。高效的胞内原位催化可以在细胞的指定位置激活一个非天然化学反应,按需合成出一个功能性分子。举例来说,如果大分子催化剂可以对细胞有选择性,那么一个抗癌分子就可能只在癌细胞内被合成出来并生效。此外,对于一些难以进入细胞的小分子或者大分子也可以使用胞内合成的方式将它们引入细胞,从而避开细胞摄取的问题。因此,尽管困难重重,国际学术界仍对细胞内原位催化这一课题兴趣浓厚。从2006年Streu和Meggers以小分子钌催化剂为例提出胞内催化反应这个概念以来,该领域即在重重困难中缓慢的发展,至今仍然大部分限制在钯、钌和铜催化的反应上,并往往需要较高的浓度方可达到胞内催化的目的。胞内催化技术作为化学生物学的重要工具,一些关键的技术难题仍然存在,如催化剂在生物环境下对常见小分子(如氨基酸等)的稳定性、大分子骨架对小分子底物的选择性和反应性影响的控制、催化剂的生物相容性等,大量问题亟待解决。近几年来,这个小领域的研究成果呈井喷式呈现,无论是修饰的天然骨架还是人工合成的大分子骨架,都在模型工作中展示出了不错的效果和潜力。这个领域有望在几年内成为化学生物学和有机化学交叉方向中最热门的小方向之一。
本项目希望结合胞内催化领域和高分子领域工作的一些基础,制备结构和性质与酶有一定相似性的大分子催化剂或其他的胞内催化剂递送体系。它们可以是由多条以树状聚合体作为侧链的刷型大分子有序组装而成并负载金属催化中心的“刷臂星型高分子”高效催化剂,也可以是由功能DNA组成的球形核酸类催化体系,或者是将催化剂递送在细胞膜上的膜融合催化体系。我们将系统研究这些类型的催化系统在胞内原位催化的表现与应用。无论是大分子仿酶催化剂还是球形核酸催化剂,它们都可以具有因外部亲水结构带来的高水溶性和高生物兼容性,也具有位于内部的疏水空腔,有望在保证催化中心更为稳定的前提下,在一定程度上仿照酶的催化机理来高效催化疏水小分子之间的反应。目标催化剂的结构特点使得该体系有望解决以往胞内催化的催化剂细胞毒性、进入细胞能力和催化活性不可兼得的问题。而催化剂递送体系也可以将活性中心“放置”在合适的地方,令它们在“舒适”的环境中发挥自己的催化功能。无论是哪一种系统,其催化剂的制备策略都允许采用通过“保持体系架构、更换催化中心”的方法来使得用同一种载体构建多效、多功能催化体系成为可能。
Design, synthesis and application of multi-mechanism antimicrobial polymers
Although the invention of antibiotics in the 20th century had doubled the life expectancy of humans, bacteria infection is still an important problem worldwide. For example, tuberculosis is a common infectious disease with high mortality rate especially in the developing countries, ranking in the top 10 reasons among all the human deaths on earth. Even in developed countries and regions, large-area infection and multi-drug resistant bacteria infection are still causing many deaths in hospitals. The wide use of antibiotics is also causing more and more severe problems from antibiotic resistance, leading to limitations of the use of many antibiotics in clinical trials, further promoting the need for the development of new antibiotics.
As a hot research area in the recent years, antimicrobial polymers hardly generate any drug resistance. Nonetheless, due to their low therapeutic index, there has not been any case in this category approved by the FDA. In contrast to most small molecular antibiotics that use a “target-and-inhibit” mechanism, the bacteria-killing effect from antimicrobial polymers solely rely on membrane disruption by inserting their positively charged hydrophobic chains into the lipid bilayers of bacteria membrane. Because this process is purely phyiscal and the synthesis of membrane involves so many biochemical processes, it is extremely difficult for bacteria to evolve from random mutations to gain resistance to such a killing process. However, since lipid bilayers also exist in mammalian cells, it is also difficult for antimicrobial polymers to gain enough selectivity toward bacterial membranes, leading to relatively low therapeutic indices. Although it is possible to improve therapeutic index by synthesizing and screening different macromolecular structures, this approach is significantly limited by the similarity of mammalian and bacterial cell membranes.
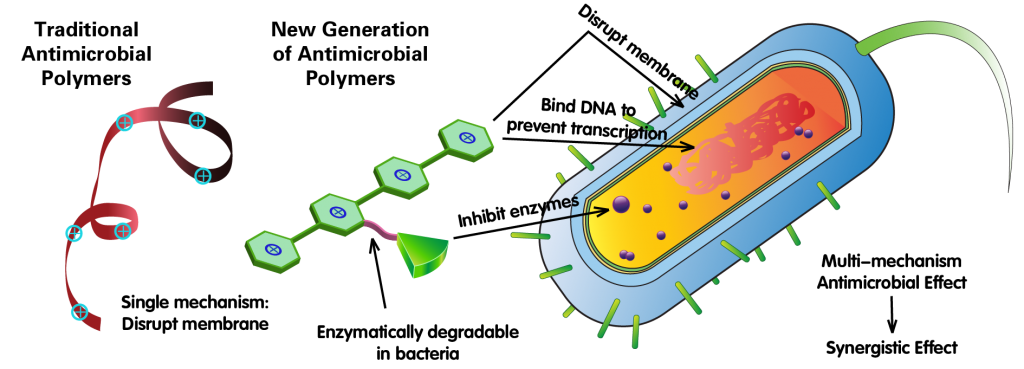
One additional advantage of antimicrobial polymers is that they can change the permeability of membranes. On the one hand, due to the disruption of membrane by the polymers, permeability increase of bacterial membranes can occur, allowing small molecule antibiotics and macromolecules to enter the bacteria, giving a potential synergistic effect. On the other hand, antimicrobial polymers can enter mammalian cells with ease, killing intracellular bacterial pathogens. This can be extremely important for some persistent disease with intracellular pathogen involved, such as tuberculosis.
Here we aim to introduce new antimicrobial mechanisms for cationic, antimicrobial polymers, in addition to their traditional membrane disruption mechanism, making them a new generation of antimicrobial complex with mixed mechanism of actions. Such new macromolecules should also enter infected mammalian cells to kill intracellular bacteria, leading to more thorough action and reduce the needed treatment time for diseases caused by intracellular bacteria. We would also like to explore the relationship between macromolecular structures and their antimicrobial effects, laying a theoretical foundation for future development of new antimicrobial materials.
尽管20世纪中抗生素的发明使人类的平均寿命整整提高了一倍以上,细菌感染仍是世界范围内的重要问题。例如,结核病是世界范围内高发的、致死率较高的重大传染病,发病率和致死率在发展中国家中尤为突出,在全球致死因素中仍然高居前十。在医疗水平较高的发达国家和地区,术后大范围感染或多重抗药性细菌造成的感染仍然很容易造成死亡。此外,抗生素的大范围使用也使得细菌抗药性的问题越来越严重,这对一些抗生素在临床上的应用产生了一定的限制,也令新型抗生素的研究工作迫在眉睫。
作为近年的热门研究方向之一的抗菌高分子具有不易产生耐药性的优点,但因治疗指数低,目前还没有被美国FDA批准的例子。与大部分小分子抗生素针对特定靶点的抗菌机理不同,抗菌高分子的主要作用机理为依赖自身的疏水链和正电荷结构,插入细菌的磷脂双分子膜之中,对膜的物理结构及动态平衡产生破坏从而杀死细菌。由于该杀菌机理是一个纯物理过程,而膜的产生又涉及到非常多的生物化学过程,因而抗菌高分子很难产生抗药性。然而,由于哺乳动物细胞也有磷脂双分子层的细胞膜,尽管其结构与细菌的细胞膜略有差别,但仍然或多或少会被抗菌高分子所影响破坏,产生细胞毒性。因此,抗菌高分子的治疗指数一般相对偏低。通过筛选优化抗菌高分子的结构可以提升其对细菌细胞膜的选择性进而提高治疗指数,但仍然有很大的局限性。抗菌高分子的另外一个特点是其对细菌细胞膜可穿透性的改变。由于抗菌高分子会对细胞膜造成物理性破坏,膜上会产生孔洞或裂隙,使膜的通透性大大增加。在膜被破坏之后,抗菌高分子往往可以进入细菌内部,也可以使其他的抗生素更容易通过细菌的细胞膜进入其内部,增强药效,即协同效应(Synergistic effect)。同理,抗菌高分子也可以通过同样的机理进入哺乳动物细胞内部,有望杀死细胞内部的细菌,这对于一些疾病(如肺结核)的治疗有非常重要的作用。
本项目力图在传统抗菌高分子的杀菌机理上引入全新的杀菌机理,使高分子成为新型的复合机理抗菌化合物,通过多个机理的协同作用,达到提高活性、降低毒性,并保持传统抗菌高分子低耐药性特点的效果,从而获取新一代多机理抗菌高分子。该抗菌高分子还可进入被感染的哺乳动物细胞,杀死其内部的细菌(Intracellular bacteria),因而具有更彻底的杀菌效果,有望降低治疗时间,提高治疗效率。项目也将进一步探究高分子结构与其抗菌效果之间的联系,为新型大分子抗菌药物的发展提供重要理论依据。
Design, synthesis and evaluation of novel polymeric vectors for gene delivery (For master and undergraduate students only)
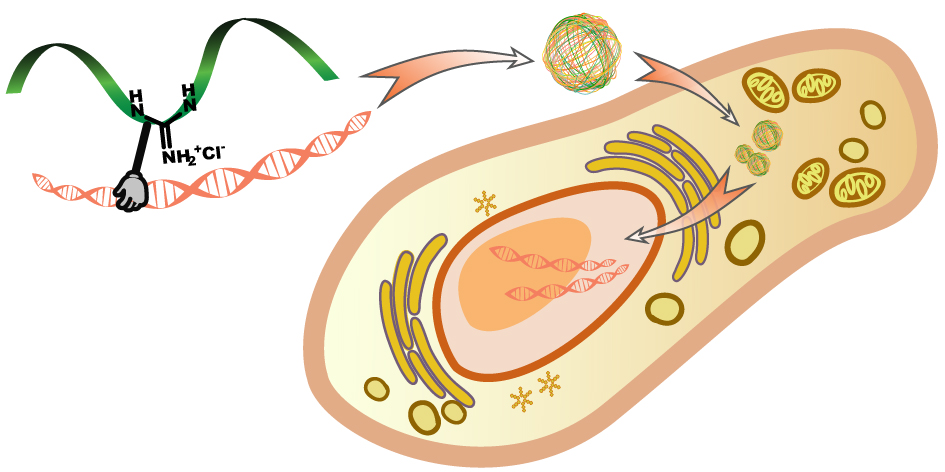
Gene delivery vectors are scaffolds that can pack DNA/RNA and deliver them into target cells. Getting the right gene into cells and making the cells expressing these genes is one of the key steps in generating disease models, biosynthetic systems, cellular models. Therefore, such delivery vectors have been playing a key role in chemical biology and molecular biology research. Polycations are a family of macromolecular gene vectors that pack DNA using electrostatic interactions with the negatively charged phosphates. These polymers can form polyplexes with DNA, get endocytosed by the cells, and eventually release the DNA cargo into the cells and allow them to be expressed. Structures of polycations can vary, including but not limited to polyethyleneimines (PEI), polypeptides, and quarternized amine-bearing polyesters, chitosan derivatives. Usually for the polymers, higher molecular weight and charge density increase DNA packing and delivery efficiency, but at the same time lead to higher cytotoxicity and hindered DNA release in cells. Based on the above considerations, we aim to integrate DNA-binding moieties onto the polymers, granting them higher DNA affinity without increasing charge density and molecular weight, so that the biocompatibility of the polymers can be improved. In addition, by using polymers with triggered degradability, it is possible to achieve smart release of DNA inside cells, leading to higher transfection efficiency without compromises on the biocompatibility of the gene vectors.
基因递送载体(Gene delivery vector/vehicle),是指用于装配基因,将其导入到靶细胞或相应的宿主细胞,使基因进行表达所必须的介质。将适当的基因引入细胞并令其表达,不仅是化学生物学和分子生物学中建立生物合成系统、细胞模型、疾病模型等的关键步骤,也是治疗众多基因疾病和各种癌症的方法之一。阳离子多聚物(Polycation)是一类常用的基因递送载体,借助其电荷密度来吸附负电性的DNA分子,形成复合体,然后粘附到细胞表面并被细胞内吞,从而使质粒表达。这些多聚物包括人工合成的聚乙烯亚胺、多肽、阳离子化聚酯等,也包括天然的多聚物如壳聚糖、胶原、阳离子多聚肽等。这类阳离子型高分子的分子量、表面电荷是决定转染效率的关键因素,但也是导致其细胞毒性的主要原因。电荷对高分子-DNA复合物的进胞效率起着促进作用,但同时也会增大细胞毒性,并影响DNA在胞内的释放。鉴于此,课题组希望通过引入DNA亲和基团(非电荷类相互作用),来使得该类型的高分子获得对DNA更高的亲和力,从而可以保证较低的用量,降低毒性。在此基础上,通过引入可控降解基团,做到DNA在胞内的智能释放,从而进一步提升基因递送的效率和基因载体的生物相容性。